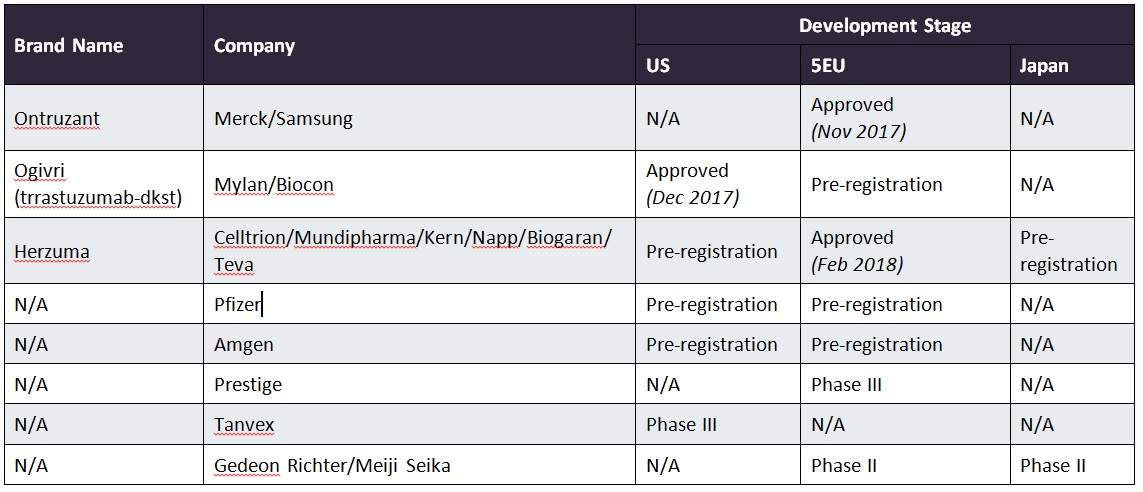
Celltrion and Teva's Herzuma (trastuzumab-pkrb- Herceptin biosimilar) Receives FDA's Approval for HER2-Overexpressing Breast Cancer

Teva two: FDA approves Celltrion-made Herceptin biosimilar - BioProcess InternationalBioProcess International

Say hello to Roche's worst-case scenario: Teva's Rituxan biosim set to launch in U.S. | Fierce Pharma